Research Interest
Different from the peripheral nervous system (PNS), axons in the adult mammalian central nervous system (CNS) usually do not spontaneously regenerate after injury. The failure of axon regeneration is one of the major obstacles for functional recovery after neurotrauma such as spinal cord or optic nerve injury. Our goal is to establish a research program to study axon regeneration at the molecular, cellular, and system levels, and contribute new knowledge to the field. Our research group has made several major discoveries on cell signaling pathways and mechanisms that regulate axon regeneration. These findings provide an opportunity to study the function of regenerating axonal tracts, rebuild disrupted circuitries, and develop therapeutic strategies after CNS injury. Our work focuses on the following areas (with some of our published research results):
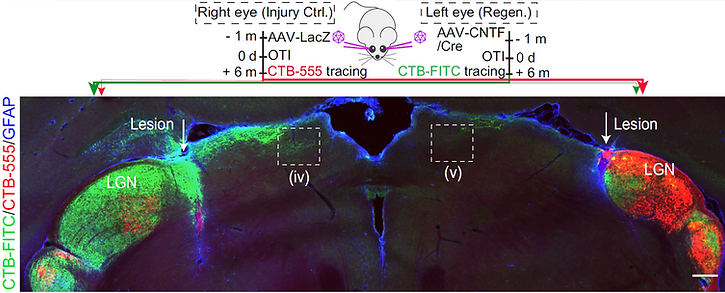
2. The role of neuronal activity in axon regeneration.
Pten inhibition activates mTOR and promotes corticospinal tract regeneration after spinal cord injury, indicating its potential as a therapeutic strategy. However, it also activates additional pathways that may contribute to its oncogenic activity. We found that subtypes of intrinsically photosensitive retinal ganglion cells (ipRGCs) in mice maintained high mammalian target of rapamycin (mTOR) levels after axotomy and that the light-sensitive GPCR melanopsin mediated this sustained expression. Melanopsin overexpression in the RGCs stimulated axonal regeneration after optic nerve crush by up-regulating mTOR complex 1 (mTORC1). Our work identifies a mechanistic link between axon regeneration and neuronal activity in vivo and provides an intrinsic factor that can be further exploited to promote neural repair after injury. (Published in PNAS 2016)
Area I: Understand the mechanisms underlining the differential axon regenerative capacity between CNS and PNS neurons
1. Genetic evidence that mTOR signaling regulates axon regeneration in adult peripheral neurons.
Neuronal mTOR activity is crucial for the regenerative ability of mature neurons in the adult central nervous system (CNS), but its impact on peripheral sensory neurons (PNS) after injury remains unknown. Using genetic approaches, we found that knocking out mTOR in DRG neurons suppressed axon regeneration induced by conditioning lesions. This provides genetic evidence that mTOR activity contributes to intrinsic axon growth capacity in adult peripheral neurons after injury. (Published in eNeuro 2016)
3. Neuronal glycerolipid metabolism determines the extent of axon regeneration.
It's still not clear how adult neurons organize lipid metabolism to regrow axons. We discovered that decreasing neuronal lipin1, an important enzyme that manages the proper production of glycerolipids through the glycerol phosphate pathway, helped axons regenerate after damage to the optic nerve. Our research shows that lipin1 and DGATs play a key role in controlling the metabolism of glycerolipids in neurons. It also suggests that shifting neuronal lipid production away from TG production and toward PL production may help axon regrowth. (Published in Neuron 2020)


4. Neuronal and non-neuronal Innate immune responses in PNS and CNS axon regeneration.
The coordination mechanism of neural innate immune responses for axon regeneration is poorly comprehended. We demonstrate that neuronal deletion of protein tyrosine phosphatase non-receptor type 2 (Ptpn2) sustains IFNγ-STAT1 activity in retinal ganglion cells (RGCs) to promote axon regeneration following injury, independent of mTOR or STAT3. Our research also demonstrates that injured PNS axons can direct the environmental innate immune response for self-repair and that the neural antiviral mechanism can be utilized to promote axon regeneration in the CNS. (Published in Neuron 2023)


Area II: Promote axon regeneration after spinal cord injury
1. Pten deletion promotes CST regeneration after chronic spinal cord injury.
Spinal cord injury (SCI) often leads to permanent functional deficits due to the failure of axon regeneration. Efforts have been made to promote axon regeneration in the spinal cord, but the ability to reverse injury-induced down-regulation of growth capacity in adult CSMNs and regenerate chronically injured CST axons remains elusive. Our study suggests that modulating Pten/mTOR signaling in adult corticospinal motor neurons can promote the sprouting of uninjured CST axons and enable the regeneration of injured axons past the lesion in a mouse model of severe spinal cord injury. We developed a strategy to modulate Pten/mTOR signaling in adult corticospinal motor neurons in the post-injury paradigm. It not only promoted the sprouting of uninjured CST axons but also enabled the regeneration of injured axons past the lesion in a mouse model of severe spinal cord injury, even when treatment was delayed up to 1 year after the original injury. The results considerably extend the window of opportunity for regenerating CST axons severed in spinal cord injuries. (Published in Journal of Neuroscience 2015)
Video showing continuous spinal cord slices with regenerated axons from ventral to dorsal side
pS6/GFP/Tuj1 staining of lipin KD Sample


2. Lipin1 Knockdown promotes descending CST and ascending sensory axon regeneration after spinal cord injury.
Our previous studies have identified the regulatory role of glycerolipid metabolism in axon regeneration through phospholipid (PL) synthesis, the key components of cell membranes. Yet, it remains unclear whether glycerolipid metabolism also coordinates cell signaling pathways to drive CNS axon regeneration. In our latest study, we demonstrate that Lipin1 overexpression inhibits axon regeneration mediated by mTOR and STAT3 activation, while Lipin1 knockdown facilitates axon regeneration through the activation of both signaling pathways. Our findings unveil the interplay between Lipin1 and cell signaling pathways in the regulation of axon regeneration and highlight the conserved role of Lipin1 in regulatng the regeneration capacity of both CNS and PNS neurons. (Published in PNAS 2024)

1. Regenerated retinal axons reform active synapses with SCN neurons after pre-chiasm optic nerve lesion.
After CNS injury, investigating axon regeneration aims to restore function. We created an injury model to test if many regenerating retinal axons can reinnervate the target location and form synaptic and functional connections with relevant neurons. This model lets us evaluate visual and non-visual function recovery following injury. We started by eliminating Pten and Socs3 in retinal ganglion cells and found that retinal axons strongly reinnervate the hypothalamus following pre-chiasm ablation. Many axons projected ectopically surrounding the suprachiasmatic nucleus (SCN) core, indicating aberrant innervation. We also found that axons connect SCN neurons with existing circuitry using trans-neuronal tracing. Our findings suggest that suppressing Pten and Socs3 in damaged neurons increases intrinsic growth capacity and axonal reinnervation and rewiring. (Published in Neurobiology of Disease 2015)
Retinal axons regenerate into the SCN region

Area III: Promote regenerated axons to make functional reconnection and recovery after CNS injury
2. Cell-type- and target-specific axon regeneration partially restores the pupillary light reflex after optic tract lesion.
The pupillary light reflex (PLR) is a well-documented classical circuit mediated by the olivary pretectal nucleus (OPN) that provides a simple system to understand axon regeneration and reconnection. We successfully executed an optic tract lesion between the lateral geniculate nucleus (LGN) and OPN using forceps, without removing part of the cortex. We then established a pre-OPN intracranial optic tract injury model and investigated the potential for retinal ganglion cell (RGC) axons to regenerate and reinnervate brain nuclei after pre-OPN injury. , this study establishes a pre-OPN intracranial optic tract injury model to investigate axon regeneration and functional reconnection in the PLR circuit. We successfully demonstrated that RGC axons could regenerate and reinnervate the OPN, leading to partial recovery of the PLR. We also explored combination strategies to accelerate recovery and further increase PLR responses, providing a foundation for future research on axonal rewiring and recovery after adult CNS injury. (Published in Nature Communications 2025)